First Human Receives Neuralink Brain Implant, ‘Recovering Well’
Elon Musk announced this afternoon that Neuralink, his neurotechnology company, has successfully implanted its first brain-computer interface (BCI) device in a human patient. The individual who received the implant is “recovering well,” said Musk, adding initial results are indicating effective neuron spike detection.
This significant milestone follows Neuralink’s statement in September 2023 about receiving approval to commence its first-in-human clinical trial. The trial, named PRIME Study (Precise Robotically Implanted Brain-Computer Interface), is a pivotal investigational device trial for Neuralink’s fully-implantable, wireless BCI, known as the N1 implant, along with its surgical robot, the R1.
The first human received an implant from @Neuralink yesterday and is recovering well.
Initial results show promising neuron spike detection.
— Elon Musk (@elonmusk) January 29, 2024
The PRIME Study is designed to evaluate the safety of the N1 implant and the R1 robot, as well as to assess the initial functionality of the BCI. The primary objective is to enable individuals with paralysis to control external devices, such as computer cursors or keyboards, using their thoughts alone.
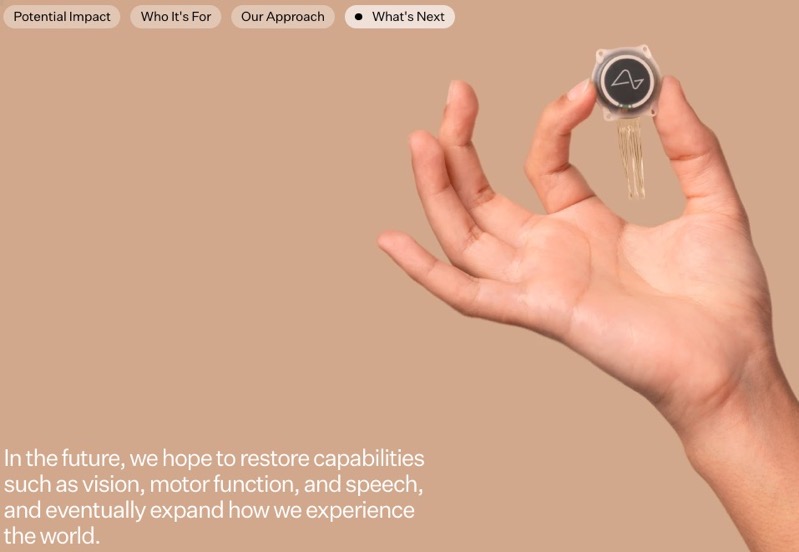
During the trial, the R1 Robot surgically places ultra-fine, flexible threads from the N1 Implant in the brain region that controls movement intention. Once implanted, the N1 is cosmetically invisible and intended to record and transmit brain signals wirelessly to an application that decodes movement intentions.
This groundbreaking study, conducted under the investigational device exemption (IDE) awarded by the FDA in May 2023, represents a critical step in Neuralink’s mission to develop a generalized brain interface. The technology aims to restore autonomy to individuals with unmet medical needs, especially those suffering from quadriplegia due to cervical spinal cord injuries or amyotrophic lateral sclerosis (ALS).
Neuralink has also opened a Patient Registry for those interested in participating in current and future clinical trials. This registry is primarily aimed at individuals who meet the specific criteria for their ongoing studies. Musk’s announcement underscores Neuralink’s commitment to advancing neurotechnology and offers hope for new therapeutic possibilities for people with severe mobility impairments.