
FDA Approves Neuralink’s Landmark Human Clinical Trials
Elon Musk’s cutting-edge neurotechnology company, Neuralink, announced on Thursday that it has secured approval from the U.S. Food and Drug Administration (FDA) to kickstart its first human clinical trial.
This milestone marks a significant leap forward in Neuralink’s quest to build advanced interfaces between the human brain and artificial intelligence. Back in March, it was rumoured Neuralink had approached the Barrow Neurological Institute, a Phoenix, Arizona-based neurological disease treatment and research organization, to help conduct human trials. It’s unclear if this is the final outcome.
With the FDA’s approval, a culmination of tremendous efforts and close cooperation between the Neuralink team and the regulatory body, the company is poised to bring its revolutionary technology from the lab to the homes of people who need it most.
Neuralink’s mission is deeply rooted in the transformative potential of brain-computer interfaces. As the company explains, “Brain-computer interfaces have the potential to change lives for the better. We want to bring this technology from the lab into peoples’ homes.”
We are excited to share that we have received the FDA’s approval to launch our first-in-human clinical study!
This is the result of incredible work by the Neuralink team in close collaboration with the FDA and represents an important first step that will one day allow our…
— Neuralink (@neuralink) May 25, 2023
Initially, Neuralink is concentrating its efforts on enabling individuals with quadriplegia, a condition resulting in partial or total loss of use of all four limbs and torso, to control their computers and mobile devices purely through thought. This step could drastically improve the quality of life for many who are currently reliant on others for these everyday tasks.
At the heart of Neuralink’s development process is a focus on the individuals who will ultimately use their products. Emphasizing safety, accessibility, and reliability during their engineering process, the company seeks to ensure that their technology can be integrated smoothly and beneficially into users’ lives.
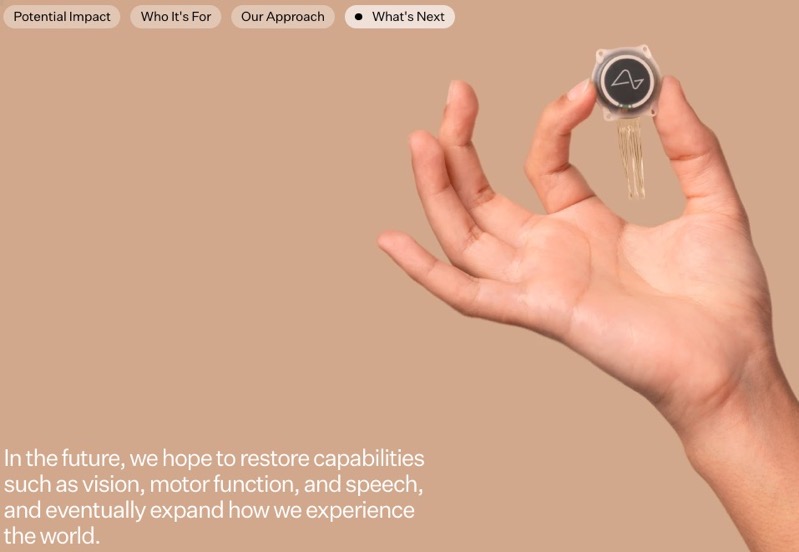
The longer-term vision for Neuralink extends even further. The company anticipates that its technology will eventually restore capabilities such as vision, motor function, and speech that may have been lost due to injury or disease. But Neuralink’s ambitions don’t stop at restoration. They see their brain-computer interface as a pathway to expand human experience itself.
Recruitment has not yet begun for this landmark clinical trial, but Neuralink has promised to release more information soon. As the world watches with keen interest, Neuralink’s first human trials promise to usher in a new era of technological interaction and potential medical miracles.